Our Approach
Prevention of Metastases and Ending Relapse
Cancer has traditionally been treated with surgery, cytotoxic chemotherapy, and/or radiotherapy.1 The past decades have seen the emergence of numerous immunomodulating drugs that use the power of the body’s own immune system to prevent, control, and eliminate cancer.2 Despite the promise of these immunotherapies, they are effective only in a select group of cancers and usually in a minority of patients with those cancers.3 In addition, resistance develops to many cancer immunotherapy treatments that target single molecular mutations or cancer pathways, so they have only modestly affected survival in some cancers.3
While immunomodulating therapies have shown promise in improving outcomes for hematological cancers, a significant unmet need remains in the treatment of solid tumors.4 Solid tumors represent approximately 90% of adult human cancers.5 In addition, metastatic disease is responsible for 90% of deaths from solid tumors.6
There is a large unmet medical need for the prevention and suppression of metastatic disease, which ultra-high-concentration nitric oxide could address.
At Beyond Cancer, our unique approach treats solid tumors and their metastases with quick, minimally invasive treatment.
Nitric Oxide for Solid Tumors
Nitric oxide is a short-lived, endogenously produced gas that has multiple mechanisms of action, depending on the concentration used. At a low concentration (<80 ppm), nitric oxide is a pulmonary vasodilator8; at a high concentration (<250 ppm), nitric oxide has antimicrobial properties9-12; and at an ultra-high concentration (>10,000 ppm), nitric oxide has been reported to show anticancer properties by activating innate and adaptive responses of the immune system.13

The use of ultra-high concentration nitric oxide for cancer treatment can be achieved only by delivery to the tumor site. At these concentrations, nitric oxide has been shown to have anti-cancer properties and potentially improving the effects of chemotherapy or radiation therapy.
Ultra-High Concentration Nitric Oxide is Necessary for Solid Tumor Regression14
At Beyond Cancer, we have developed a proprietary system for the delivery of gaseous nitric oxide at ultra-high concentrations directly into solid tumors. Based on our current preclinical findings, we are developing treatment protocols using ultra-high nitric oxide concentrations to not only partially ablate primary tumors locally but also recognize and attack distant metastases systemically via stimulation of an antitumor immune response.

Mechanism of Action
Ultra-High Nitric Oxide Therapy (UNO)
UNO in the ablation of solid tumors may result in a systemic memory immune response that will attack both primary tumor regrowth and metastases.13
Our Hypothesis: Dual Actions of UNO
Exogenous high-concentration gaseous NO (>10,000 ppm) administered directly to a solid tumor may cause local cell death, resulting in systemic exposure to tumor antigens.
Tumor antigens may trigger a systemic immune response, thereby creating a memory immune response that will recognize and attack subsequent primary tumor regrowth as well as distant metastases.
Treatment: Day 0
On treatment day 0, ultra-high-concentration nitric oxide is delivered directly into a large solid tumor for a short period of time to allow for the killing of all cells that it comes into contact with, only partially ablating the tumor.
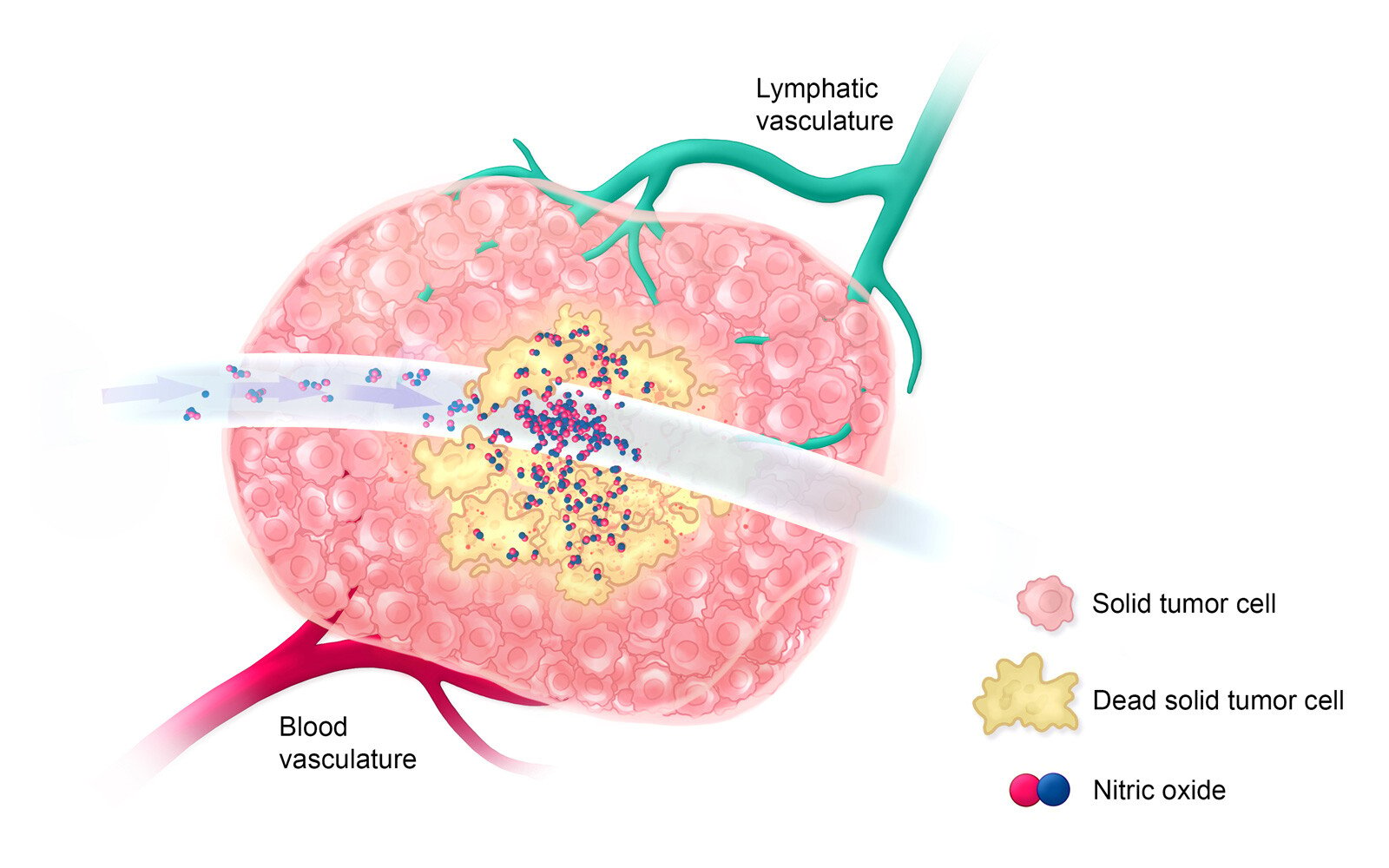
Estimated Timeline: Day 0-5
Within 5 days after treatment with ultra-high-concentration nitric oxide, antigen-presenting immune cells invade the tumor environment to detect and engulf dead solid tumor cells.
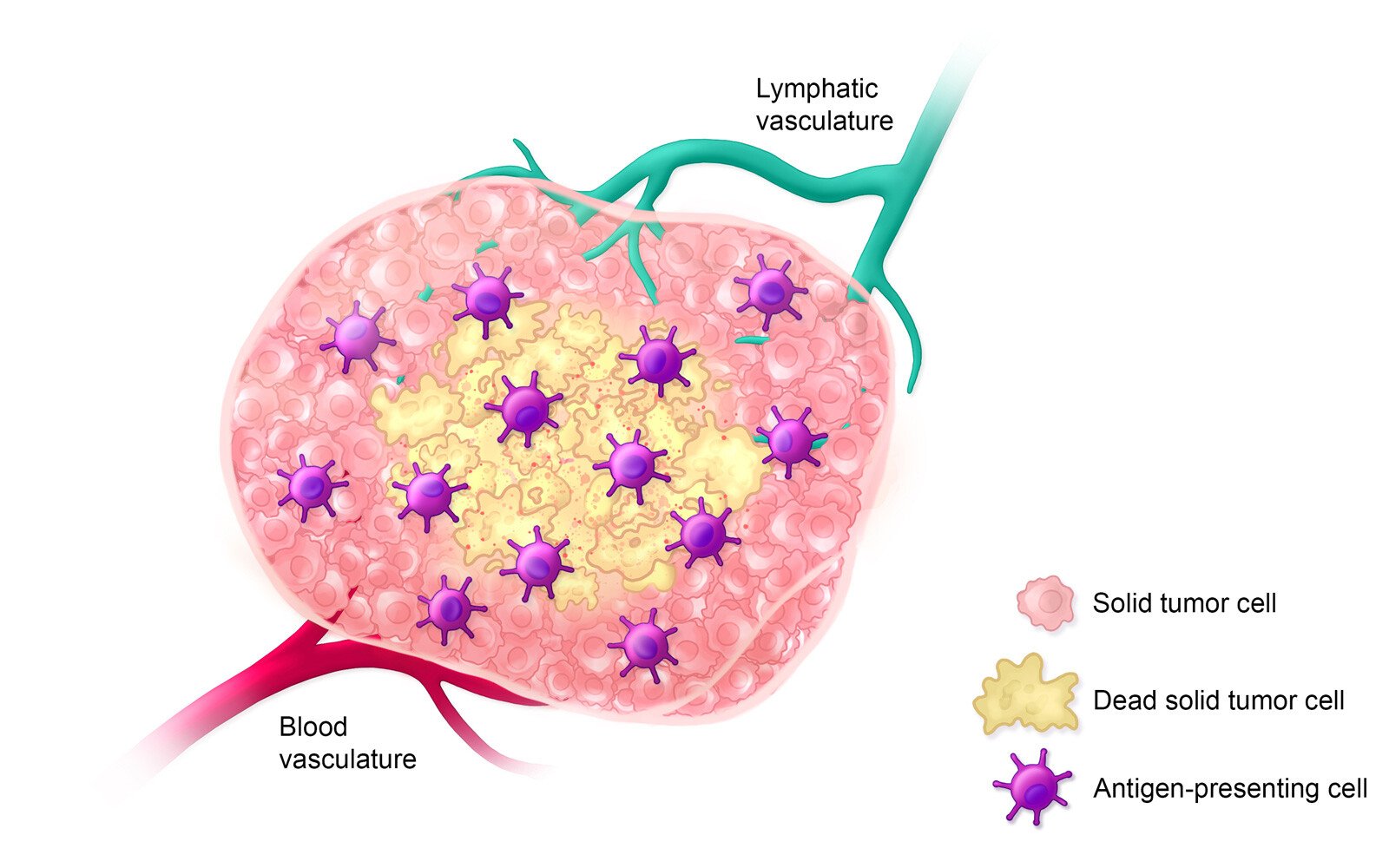
Estimated Timeline: Day 5-14
On days 5-14 after treatment, antigen-presenting cells inform the adaptive immune response by presenting antigens to immature T cells, thereby activating them.
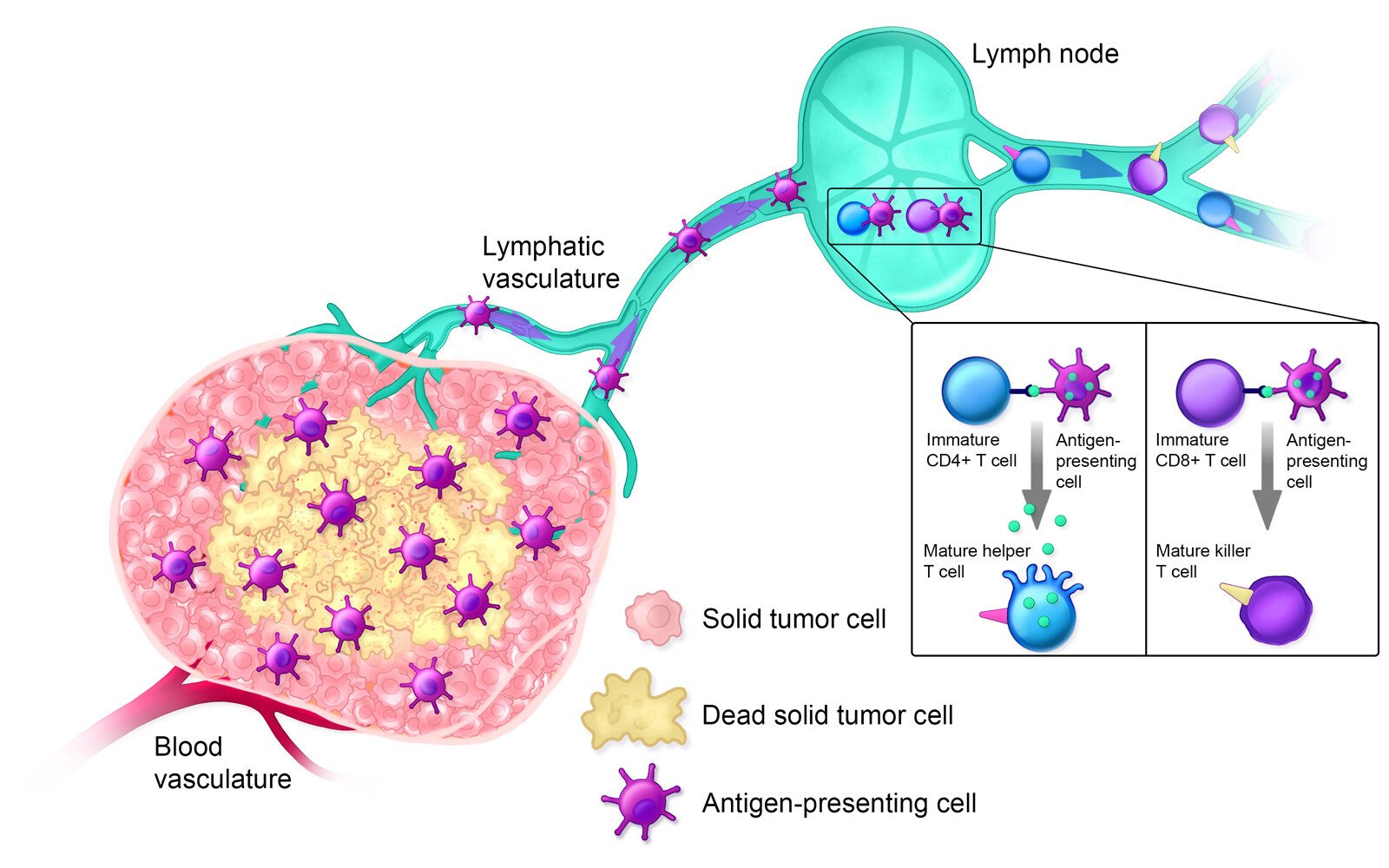

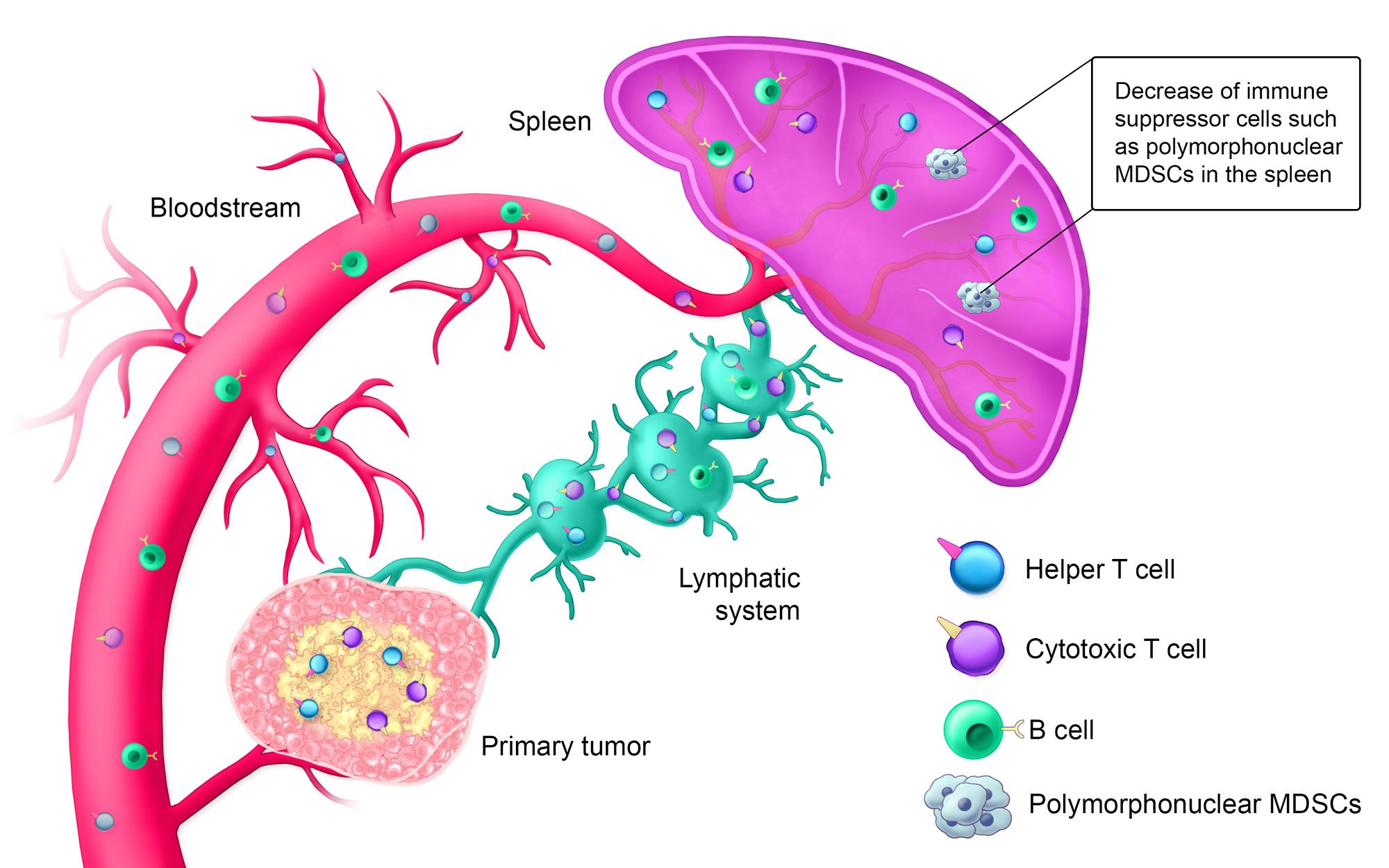
Estimated Timeline: Day 14+
Approximately 14+ days after treatment, the innate immune system is activated; helper T cells, cytotoxic T cells, and B cells are circulating in the bloodstream; and the lymphatic system is armed against the specific solid tumor type. Additionally, polymorphonuclear myeloid-derived suppressor cells (MDSCs), which suppress the immune system when tumors are present, are decreased in the spleen.


In Vitro Data
Nitric Oxide Shows a Cytotoxic Effect on Colon and
Breast Cancer Cells15
In vitro, mouse colon and breast cancer cell lines CT26 and 4T1, respectively, were exposed to gaseous NO at 150-50,000 ppm for 10-180 seconds. Cell viability was measured 24 hours later.

Significant reduction in the viability of mouse colon cancer cells after exposure to 50,000 ppm NO vs. air for 10-180 seconds.
Abbreviations: NO, nitric oxide; ppm, parts per million.
Significant time-dependent reductions in viability of mouse breast cancer cells after exposure to 150-50,000 ppm NO or air for 10-180 seconds.
In Vivo Data
Exogenous Nitric Oxide at High Concentrations Stimulates an Antitumor Immune Response in a Mouse Colon Cancer Model15
In vivo results showed that all treated colon tumor–bearing mice were resistant to a second CT26 cancer cell inoculation in a contralateral tumor challenge model.
Challenge assay: The immune response of tumor-bearing treated mice was evaluated by a challenge assay. The tumors of colon (CT26) tumor–bearing mice were treated with gaseous nitric oxide. Up to 14 days post treatment, mice were re-inoculated with CT26 cells and the percentage of tumor take was monitored.


UNO Showed Evidence of Dose-dependent Effects in Mouse Colon Cancer Model16
Challenge assay: Colon tumor–bearing mice received a single treatment with high-concentration (20,000 or 50,000 ppm) gaseous nitric oxide intratumorally for 5-10 minutes.12 Up to 21 days after nitric oxide administration to the primary tumor, all mice were re-inoculated with CT26 colon cancer cells as a challenge assay, and the percentage of tumor take and survival were monitored.
At day 45, tumor growth was observed in:
- 100% of naive mice
- 27% of mice who had received 20,000 ppm gaseous nitric oxide
- 0% of mice who had received 50,000 ppm gaseous nitric oxide


Percentage of mice who were alive at day 45:
- 25% of naive mice
- 73% of mice who had received 20,000 ppm gaseous nitric oxide
- 100% of mice who had received 50,000 ppm gaseous nitric oxide

Abbreviations: NO, nitric oxide; ppm, parts per million.
Combination Therapy
UNO + anti-mPD-1
Key results
Day 9: Tumor volumes for UNO (5 or 10 minutes) + anti-mPD-1 treated mice were smaller than either anti-mPD-1 alone or UNO alone treated mice. (p-value = 0.0005 for 10 min UNO + anti-mPD-1 combination vs anti-mPD-1 alone.)
Day 100: 53% of mice treated with 50,000 ppm UNO for 10 minutes plus anti-mPD-1 were primary and secondary tumor-free. Survival was increased when treated with 10 min UNO and anti-mPD-1. (p-value = 0.0653 for 10 min UNO + anti-mPD-1 combination vs anti-mPD-1 alone.)
Study design: CT26 tumor-bearing mice were induced with a secondary tumor two days before UNO treatment and treated with anti-mPD-1 two days before UNO treatment. Mice received 4-5 anti-mPD-1 treatments. Tumor volume and survival were monitored.


TUMOR VOLUME AT STUDY DAY 9 - DIFFERENCE BETWEEN TREATMENT GROUPSa
| Treatment Group | n | Adjusted Mean (SEM) | Adjusted Mean | 95% CI | p-value |
|---|---|---|---|---|---|
| Anti-mPD-1 | 16 | 446.28 (68.73) |
NA | NA | NA |
| NO 50,000 ppm 10 min + Anti-mPD-1 |
15 | 100.43 (68.74) |
-345.85 | (-538.09, -153.62) | 0.0005 |
aAnalysis via mixed model repeated measures (MMRM) with fixed effects for baseline tumor volume, study day, treatment by study day interaction.
Some timepoints combining data from different measurement days (day 2 = 1+2; day 5 = 4+5; day 7 = 6+7; day 9 = 8+9)


References: 1. Agarwal MB. Is cancer chemotherapy dying? Asian J Transfus Sci. 2016;10(suppl 1):S1-S7. doi:10.4103/0973-6247.182735; 2. What is cancer immunotherapy? Cancer Research Institute. Updated October 2020. Accessed September 14, 2021. https://www.cancerresearch.org/en-us/immunotherapy/what-is-immunotherapy; 3. Ventola CL. Cancer immunotherapy, part 3: challenges and future trends. Pharm Ther. 2017;42(8):514-521; 4. Kosti P, Maher J, Arnold JN. Perspectives on chimeric antigen receptor T-cell immunotherapy for solid tumors. Front Immunol. 2018;9:1104. doi:10.3389/fimmu.2018.01104; 5. Cooper GM. The Cell: A Molecular Approach. 2nd ed. Sinauer Associates; 2000. Accessed September 14, 2021. https://www.ncbi.nlm.nih.gov/books/NBK9963; 6. Fontebasso Y, Dubinett SM. Drug development for metastasis prevention. Crit Rev Oncog. 2015;20(5-6):449-473. doi:10.1615/CritRevOncog.v20.i5-6.150; 7. Choudhari SK, Chaudhary M, Bagde S, Gadbail AR, Joshi V. Nitric oxide and cancer: a review. World J Surg Oncol. 2013;11:118. doi:10.1186/1477-7819-11-118; 8. Gentile MA. Inhaled medical gases: more to breathe than oxygen. Respir Care. 2011;56(9):1341-1359. doi:10.4187/respcare.01442; 9. Saura M, Zaragoza C, McMillan A, et al. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity. 1999;10(1):21-28. doi:10.1016/s1074-7613(00)80003-5; 10. Akerström A, Mousavi-Jazi M, Klingström J, Leijon M, Lundkvist A, Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol. 2005;79(3):1966-1969. doi:10.1128/JVI.79.3.1966-1969.2005; 11. Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25(4-5):434-456. doi:10.1016/s0891-5849(98)00092-6; 12. Ghaffari A, Miller CC, McMullin B, Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide. 2006;14(1):21-29. doi:10.1016/j.niox.2005.08.003; 13. Seabra AB, Durán N. Nitric oxide donors for prostate and bladder cancers: current state and challenges. Eur J Pharmacol. 2018;826:158-168. doi:10.1016/j.ejphar.2018.02.040; 14. Carpenter AW, Schoenfisch MH. Nitric oxide release: part II: therapeutic applications. Chem Soc Rev. 2012;41(10):3742-3752. doi:10.1039/c2cs15273h; 15. Confino H, Lisi S, Golden P, et al. Gaseous nitric oxide at high concentrations is a powerful anti-tumor agent both in-vitro and in-vivo. Poster presented at: American Association for Cancer Research Annual Meeting [virtual]; June 22, 2020; 16. Confino H, Yarkoni S, Goldshtein M, et al. Nitric oxide lung cancer active vaccination. Abstract presented at: International Association for the Study of Lung Cancer 2020 North America Conference on Lung Cancer [virtual]; October 16, 2020.

